Smart Therapies for Difficult Diseases®
Our investigational immunotherapy products are tailored to each patient’s needs. We employ a “triangle offense” designed to deliver durable, complete responses in cancer and infectious diseases.
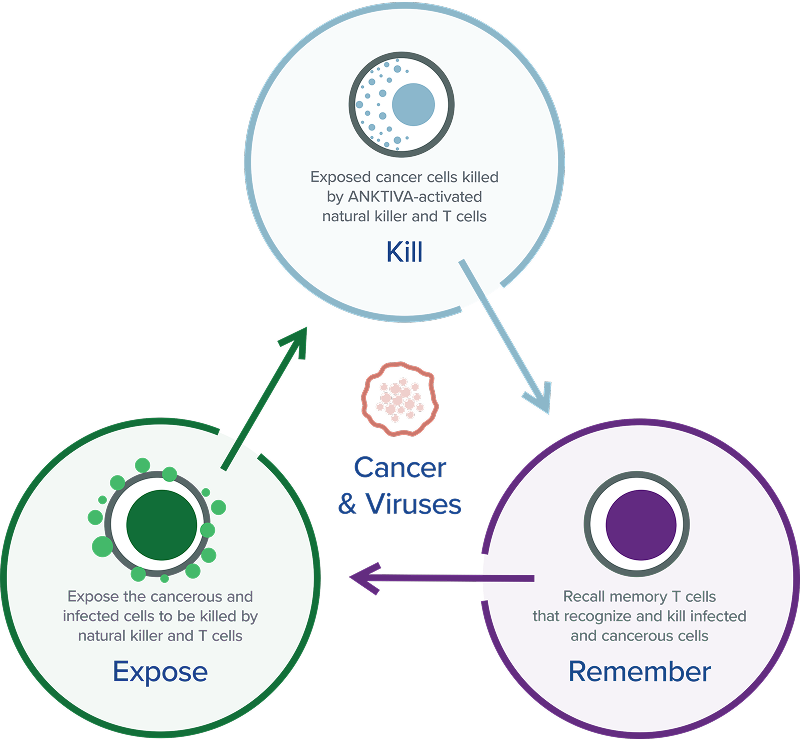
At ImmunityBio, we envision a day when we no longer fear cancer, but are able to conquer it, thanks to the biological wonder that is the human immune system. Our scientists are working to develop remarkable new therapies that harness that inherent power by amplifying both the innate and adaptive branches of the immune system, to facilitate the attack and elimination of cancerous or infected cells today while building immunological memory for tomorrow. The goal: to reprogram the patient’s immune system and treat the host rather than just the disease.

ImmunityBio
Investigational Immunotherapies

ImmunityBio is developing immunotherapies that activate both innate and adaptive immune responses to treat cancer and infectious diseases. Our approach stimulates NK cells, T cells, macrophages, and dendritic cells to create a durable immune response, aiming to outperform treatments like CAR-T or checkpoint inhibitors.
Learn more about our therapeutic areas
ImmunityBio is continuously pursuing new immunotherapies designed to attack disease by enhancing the patient’s immune system, not weakening it.
A Robust Clinical Pipeline
We are applying our science and platforms, including the development of potential cancer vaccines, to treat cancers, as well as developing immuno- and cell therapies that we believe could sharply reduce or eliminate the need for standard high-dose chemotherapy.
